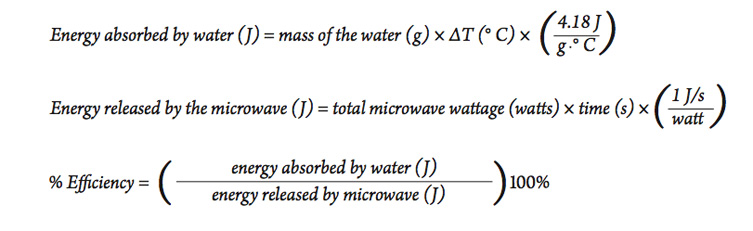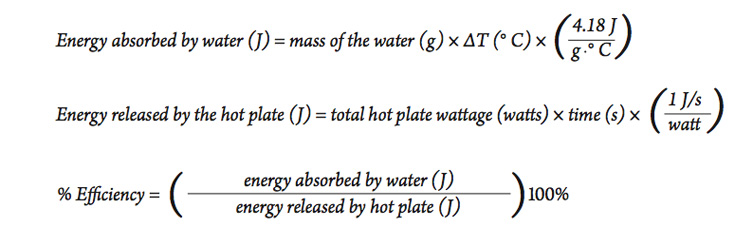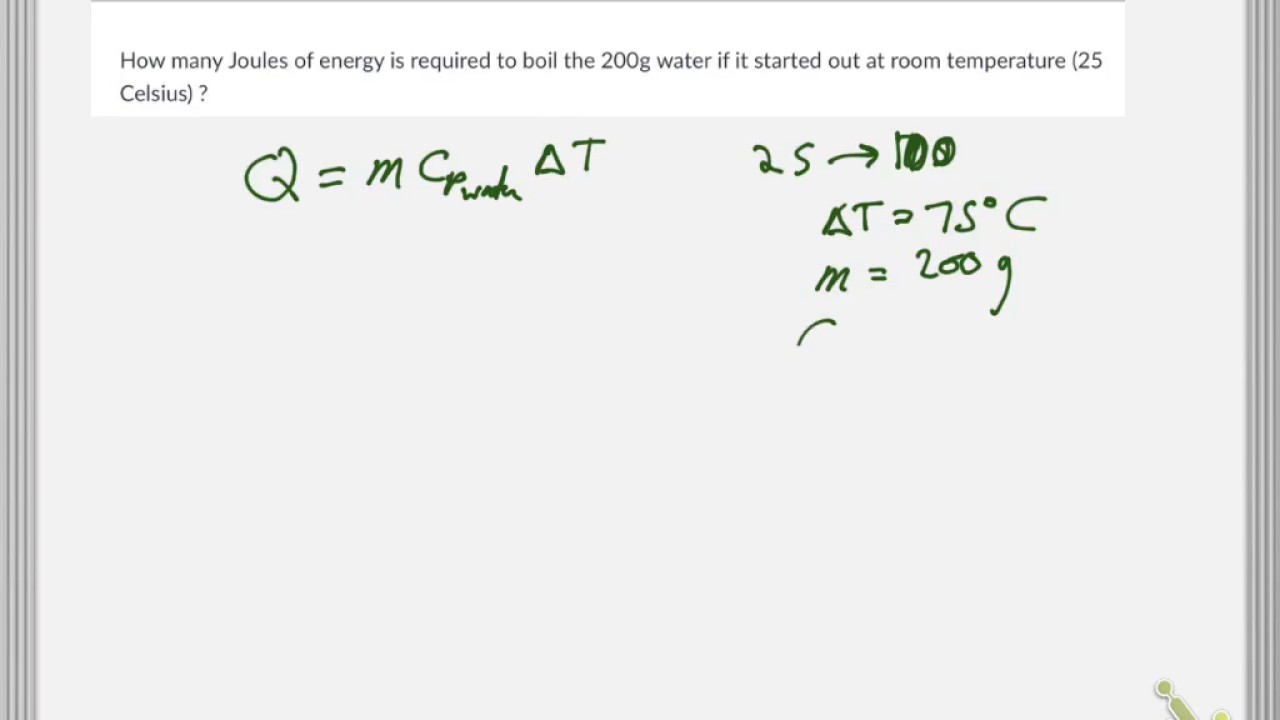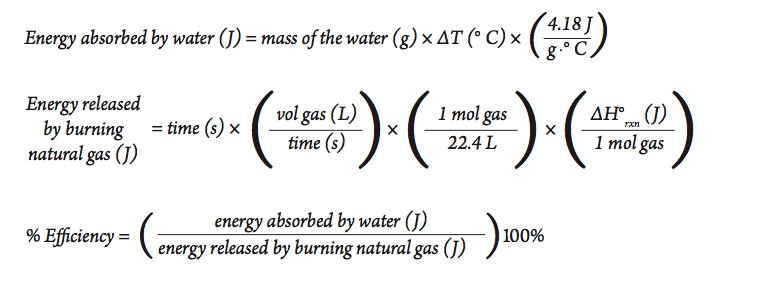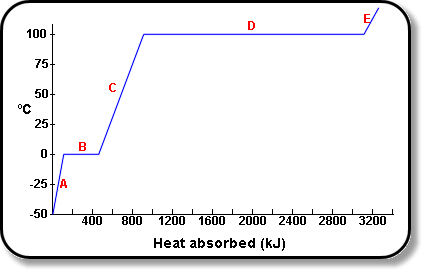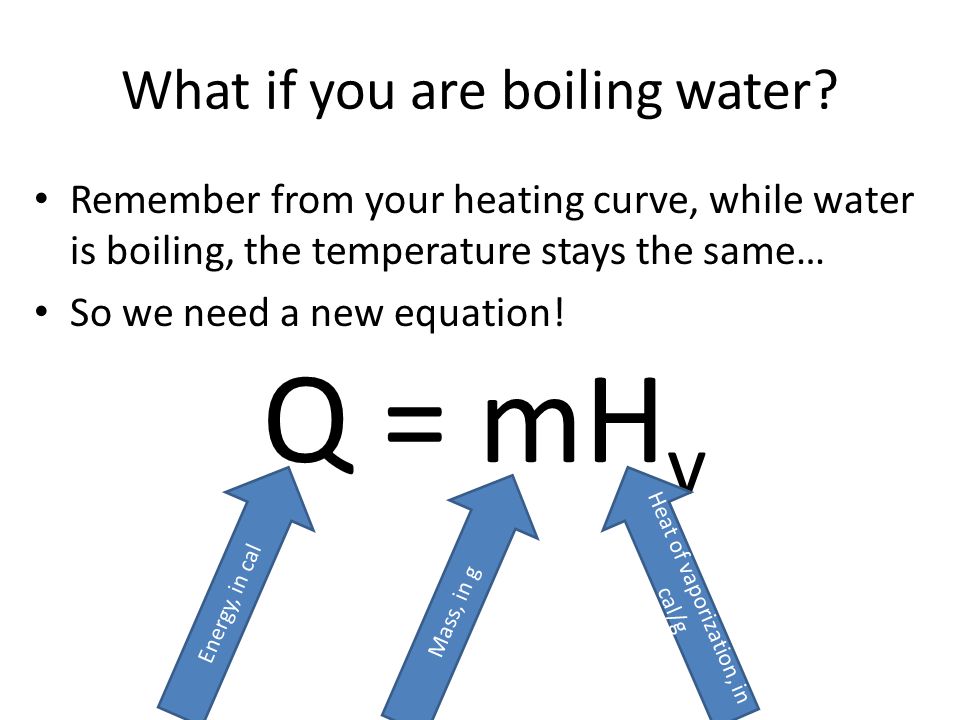
Calorie (energy) Calculations A calorie is defined as the amount of energy it takes to raise the temperature of one gram of water by one degree Celsius. - ppt download
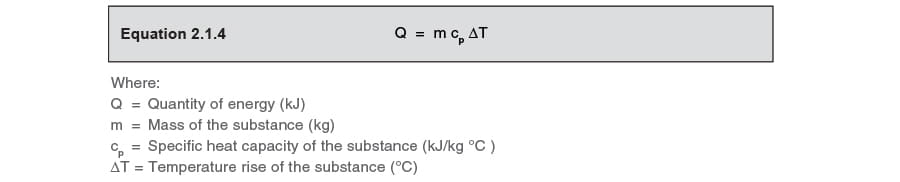
Enthalpy. Specific Heat Capacity Definition: The HEAT ENERGY required to raise the TEMPERATURE of 1kg of substance by 1 o C. e.g. for water C= 4.18kJ. - ppt download
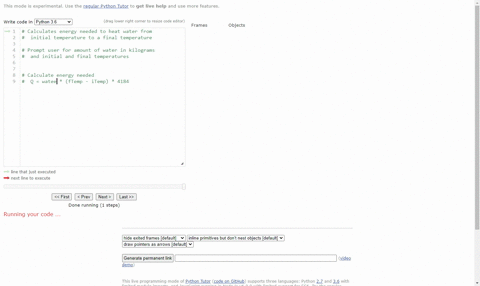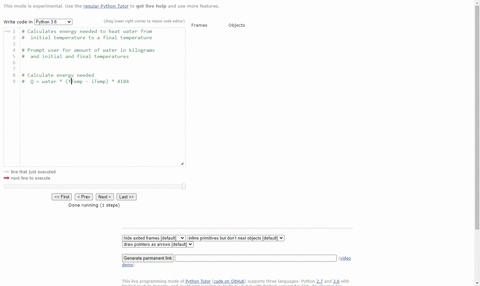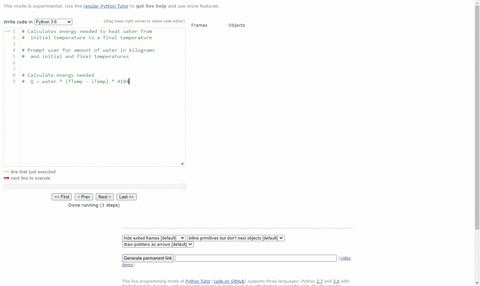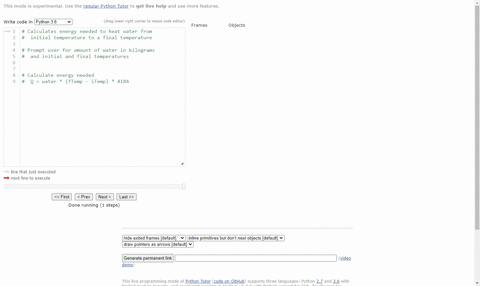
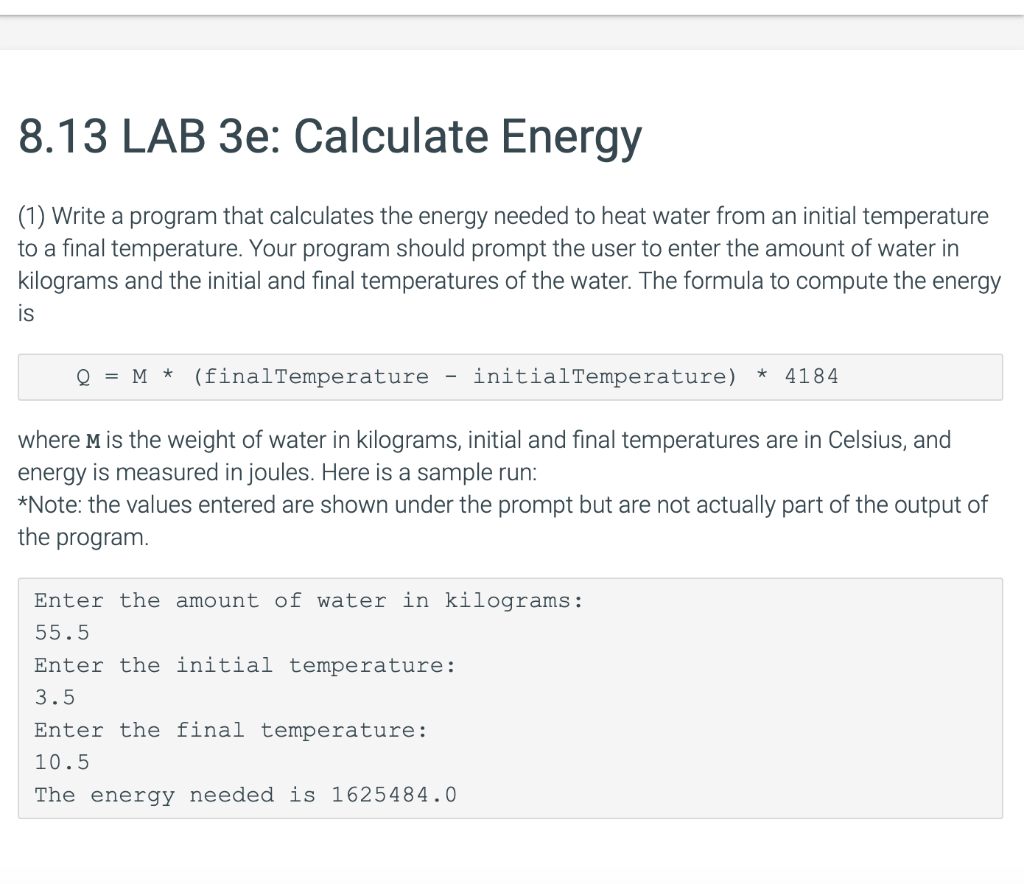
SOLVED:(Science: calculate energy) Write a program that calculates the energy needed to heat water from an initial temperature to a final temperature. Your program should prompt the user to enter the amount
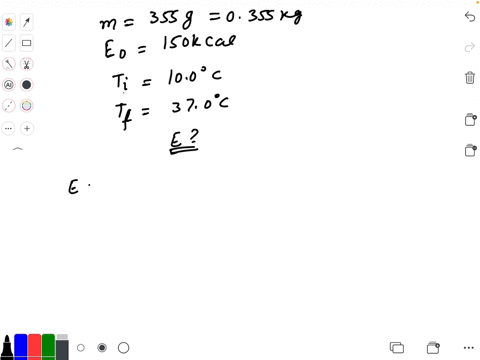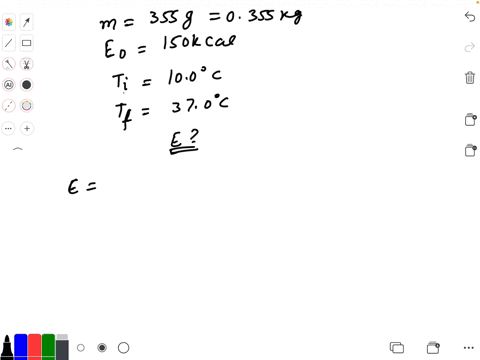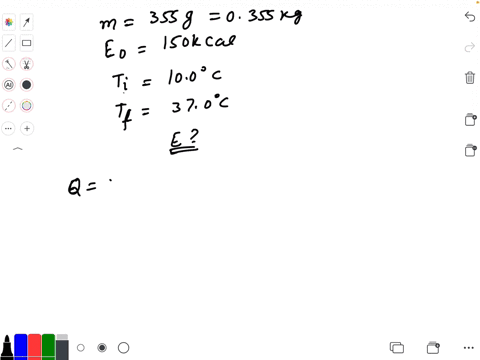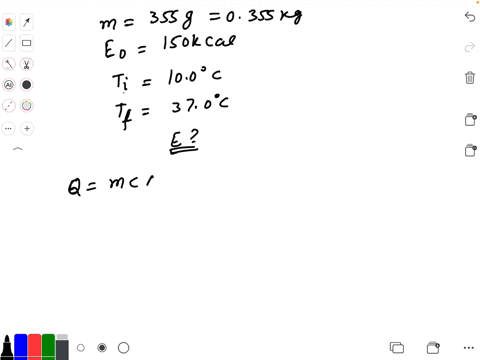
SOLVED: Calculate The Energy Needed To Heat The Cube Of Silver; With A Volume Of Cm? From 14 C To 26 Refer To The Tables Express The Heat In Calories | C&c
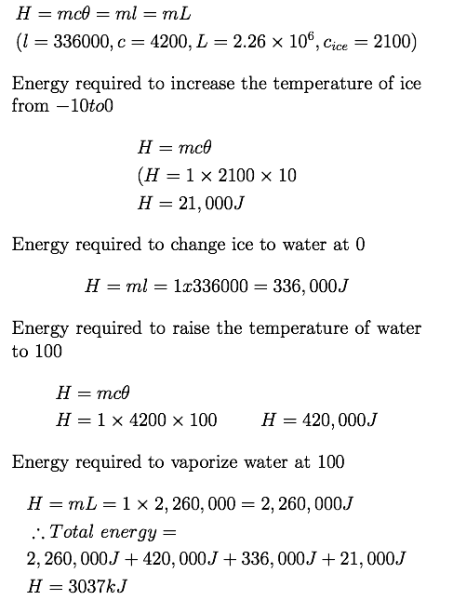
calculate the total energy required to evaporate completely 1kg of ice that is initially at... - Myschool

Calculate the heat energy required to raise the temperature of 2 kg of water from 10^@C to 50^@C. Specific heat capacity of water is 4200 JKg^(-1) K^(-1)

Calculating Heat. Specific Heat Amount of heat energy needed to raise the temp of 1 ml of a substance 1°C For water the specific heat is 4.19 J/g °C, - ppt download

SOLVED: Calculate the energy needed to heat 250.0 g of water from 25 degrees * C to 175 degrees * C . Specific heats : water=4.18 J/g^ C,steam=1.99 J/g^ C . A )